Abstract
Introduction The 2021 European LeukemiaNet (ELN) recognizes MRD relapse as ≥1 log rise confirmed on repeat testing (Heuser Blood 2021). Molecular MRD relapse (e.g. NPM1mut) precedes clinical relapse by ~3-4 months (Ivey NEJM 2016) and provides a window of opportunity for pre-emptive therapy to be delivered prior to clinical relapse. Venetoclax (VEN)-based low intensity therapy is associated with rapid achievement of clinical response in older patients (pts) with AML (DiNardo NEJM 2020 and Wei Blood 2020) but only has modest activity in relapsed/refractory AML. We hypothesized that VEN-based therapy initiated at the time of MRD relapse could be associated with improved clinical response and longer clinical benefit. We therefore conducted a prospective study to explore the efficacy of low dose ara-C (LDAC) + VEN in pts with AML and MRD relapse (ACTRN12619000746134).
Methods This multicenter phase 2 study stratified pts into either oligoblastic relapse (marrow blasts 5-15%; Group A), or molecular MRD relapse (Group B). Pts received VEN 600 mg (days 1-28, without dose ramp-up for Group B) and LDAC 20 mg/m2 sc (days 1-10). Primary objectives were morphologic or MRD response (≥1 log reduction) in groups A and B, respectively. Key secondary objectives were realization of allogeneic hematopoietic cell transplantation (HCT), relapse-free (RFS) and overall survival (OS). The study was approved by Alfred Health ethics committee (196/19). NPM1mut and other fusions (per 100 ABL1) from bone marrow were analyzed by RT-qPCR, and IDH1/2mut by Bio-RadTM droplet digital PCR.
Results A total of 48 pts were recruited (median age 67; range 18-80). Cytogenetic risk was intermediate in 91%, favorable in 4.5% and adverse in 4.5%. Most (94%) had received prior intensive chemotherapy and only 2 (4%) had received prior HCT in first remission. The median interval from AML diagnosis to study entry was 13.7 months (IQR 9, 23). At the time of study screening, 22 (46%) pts had oligoblastic disease and 26 (54%) MRD relapse. Oligoblastic relapse included pts with early progression not captured by interval MRD surveillance. Pts received a median of 3 cycles (range 1-21+) of VEN-LDAC (ongoing in 11). Reasons for treatment cessation in 37 pts were: HCT (n=19, 51%), treatment failure (n=11, 30%), adverse events (n=3), donor lymphocyte infusion (DLI, n=2) or death before response assessment (n=2). One patient (Group B, NPM1mut) was deemed not evaluable for response assessment after only receiving 9 days of therapy but included in the survival analysis.
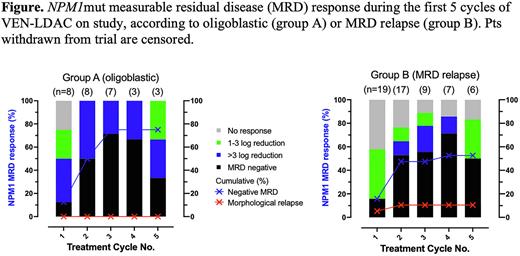
Among 22 pts with oligoblastic disease, CR/CRi was achieved in 17 (77%). Two additional pts achieved morphologic leukemia-free state and 1 had stable disease. MRD response (≥1 log reduction) among those with an evaluable marker (N=8; all NPM1 mutated) was achieved in 8/8 (100%), with MRD negative in 6/8 (75%) (Figure).
Among 25 evaluable pts with MRD relapse, MRD response (≥1 log reduction) was observed in 68% and MRD negative in 52%. Of patients with NPM1mut AML treated at MRD relapse, 13/19 (68%) achieved an MRD response and 10/19 (53%) an MRD negative response (Figure). An MRD response was observed in 2/3 with IDH1/2mut, 1/2 with core-binding factor and 1/1 with KMT2A::MLLT3.
Bridging to HCT or DLI (median time 4 months) was successful in 18/27 (67%) pts ≤70 years. With a median follow up time of 14.4 months, the median RFS was 21.5 months and not reached for OS. The estimated 18-month RFS was 63.3% (95% CI, 48.0-83.5) and OS 71.1% (95% CI, 56.5-89.4). Similar outcomes were observed for Groups A and B. Subsequent HCT (as a time-dependent covariate) was the only significant factor for RFS (HR 0.13, 95% CI 0.03-0.61) but not OS (HR 0.48, 95% CI 0.13-1.75) in a multivariate Cox regression model that included age, features at original diagnosis (NPM1, FLT3-ITD and ELN 2017 risk), time since AML diagnosis, and study cohort (A vs B). FLT3-ITD was detected in 3/7 (43%) of relapsed pts.
Conclusions In this prospective study of pts with early relapsing AML (oligoblastic or MRD relapse), VEN in combination with LDAC induced a high rate of molecular MRD response, resulting in excellent RFS and OS outcomes. Follow-up is ongoing to determine the durability of response. This novel MRD-targeting approach is being further investigated in a multi-arm, precision-based platform trial ( INTERCEPT: Investigating Novel Therapy to Target Early Relapse and Clonal Evolution as Pre-emptive Therapy in AML; ACTRN12621001265864).
Disclosures
Tiong:Servier: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Hiwase:Novartis: Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Speakers Bureau. Bajel:Abbvie: Honoraria; Amgen: Honoraria, Speakers Bureau; Astellas: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Takeda: Honoraria. Palfreyman:AstraZeneca: Honoraria, Speakers Bureau; AbbVie: Consultancy. Fleming:Novartis: Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau. Fong:Jazz: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau; Astellas: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Wei:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Employee of the Walter and Eliza Hall Institute and is eligible for a fraction of the royalty stream related to Venetoclax, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
OffLabel Disclosure:
This presentation will discuss the use of venetoclax in targeting measurable residual disease and early relapse of acute myeloid leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal